Issue Archive
Table of Contents
BLOOD COMMENTARIES
REVIEW ARTICLE
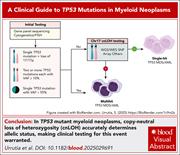
A clinical guide to TP53 mutations in myeloid neoplasms
Treating hematologists know that a finding of TP53 mutation is associated with poor prognosis in myeloid neoplasms. However, methods for detection, reporting, and criteria for what constitutes TP53-mutant status vary. Urrutia et al review the available data, provide clinically pertinent recommendations for interpretation of results in practice, and explain why biallelic aberrations are most important and how combinations of tests may be required to confirm their presence.
CLINICAL TRIALS AND OBSERVATIONS
Final analysis of the RESONATE-2 study: up to 10 years of follow-up of first-line ibrutinib treatment for CLL/SLL
Clinical Trials & Observations
With long-term follow up, registration trials can provide invaluable data about new classes of drugs, and the initial phase 3 trial of ibrutinib for first-line treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is testament to this. Burger and colleagues report that, after 10 years of follow-up, median progression-free survival is a remarkable 8.9 years, dose reduction is effective in managing many of the typical side effects, secondary leukemias are rare, and more than one-fourth of all initiating patients remain on ibrutinib.
Real-world outcomes of patients with aggressive B-cell lymphoma treated with epcoritamab or glofitamab
Clinical Trials & Observations
Epcoritamab and glofitamab are both off-the-shelf bispecific CD20×CD3 T-cell engagers that are approved by the US Food and Drug Administration for the treatment of relapsed diffuse large B-cell lymphoma (DLBCL). Brooks and colleagues report the clinical outcomes of 245 real-world patients, 70% of whom would not have met trial entry criteria, who received these drugs as a standard-of-care treatment, the majority after chimeric antigen receptor T-cell therapy. While durability of complete responses (CRs) appears consistent with clinical trial data, CR rates are lower at approximately 25%, as is progression-free and overall survival, with absence of CD20 expression portending poor response and clinical trial eligibility associated with better outcomes. These data indicate that the optimal use of these bispecific agents has yet to be defined.
IMMUNOBIOLOGY AND IMMUNOTHERAPY
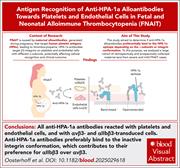
HPA-1a antibodies in FNAIT do not distinguish αvβ3 from αIIbβ3, and bind inactive integrins more strongly than active integrins
LYMPHOID NEOPLASIA
α-Ketoglutarate promotes amino acid depletion and suppresses B-cell lymphoma growth and development
The role of altered metabolism in hematological malignancies is increasingly recognized beyond specific examples such as isocitrate dehydrogenase–mutant acute myeloid leukemia. Using human diffuse large B-cell lymphoma (DLBCL) cell lines, patient samples, and Myc-driven murine B cells, Jaafar et al found that supplementation of α-ketoglutarate (α-KG) to lymphoma cells increases cell death, regardless of cell of origin or genetic characteristics. α-KG supplementation rewires branched-chain amino acid metabolism to deplete amino acids, thereby inactivating mTORC1 via perturbed lysosomal localization and suppressing lymphoma growth. These data provide clues for future clinical investigation.
MYELOID NEOPLASIA
Epidemiology, clinical features, and molecular basis of TTMV::RARA-driven acute promyelocytic leukemia
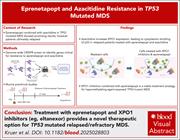
XPO1 drives resistance to eprenetapopt and azacitidine and can be targeted in TP53-mutated myeloid malignancies
Myeloid neoplasms (MN) with TP53 mutations represent a distinct disease group with dismal outcomes, reflecting resistance to existing therapies. Kruer and colleagues report that upregulation of the nuclear exporter XPO1 is central to acquired resistance in TP53-mutant MN following treatment with the combination of azacitidine and the experimental p53 reactivator eprenetapopt in clinical trials. Their associated preclinical investigations using cell lines and patient-derived xenografts support future clinical trials of existing XPO1 inhibitors in combination with eprenetapopt in patients who fail frontline therapies.
THROMBOSIS AND HEMOSTASIS
Factor IXa and factor X influence factor VIIIa stability and inactivation mechanisms in vitro and in vivo
Factor VIII (FVIII) is activated by thrombin at sites of vascular injury, and low levels cause hemophilia while high levels increase thrombotic risk. Inactivation of FVIII is thought to occur via at least 2 mechanisms. Using biochemical and in vivo studies, Morris et al provide evidence that both dissociation of its A2 domain and cleavage by activated protein C inactivate FVIII in vivo and that interactions with FIXa and FX modulate which mode of inactivation is predominant. These data are likely to be important to inform therapeutic drug design.
BLOOD WORK
ERRATUM
-
Cover Image
Cover Image
![issue cover]()
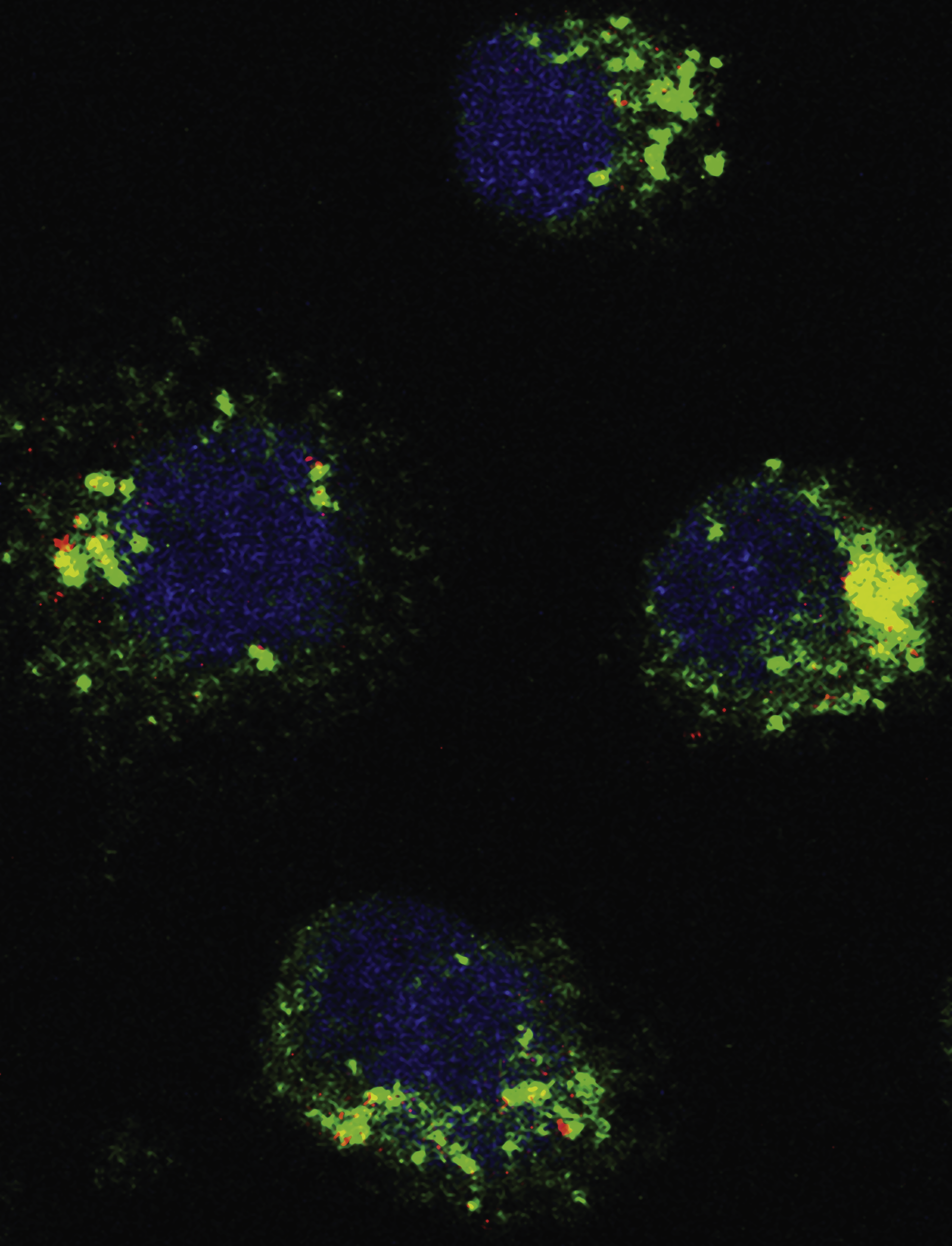
Confocal microscopy image of diffuse large B-cell lymphoma cells ectopically expressing Raptor-Rheb15 shows colocalization (yellow) of mammalian target of rapamycin complex 1 (green) with the lysosome (LAMP1; red) despite the depletion of leucine induced by α-ketoglutarate. The nucleus is shown in blue. See the article by Jaafar et al on page 2217.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals







How far can ibrutinib resonate?
Clinical Trials & Observations