In this issue of Blood, Jaafar et al1 demonstrate how metabolite supplementation restricts lymphoma growth by depleting amino acids and regulating mechanistic target of rapamycin (mTORC1) localization.
Reprogramming and rewiring of cellular metabolism are now considered a cancer hallmark.2,3 Metabolic alterations in cancer cells help support bioenergetics, biomass synthesis and the signaling, and epigenetic and gene expression changes underpinning tumor development and progression. Strategies to target cancer metabolism for cancer therapy however, have not progressed either due to toxicity to normal cells and/or lack of efficacy due to the metabolic plasticity of cancer cells. Continued investigation of context-specific metabolic vulnerabilities and the use combination strategies will better allow for targeting cancer metabolism therapeutically. The concept of hyperactivation of metabolic enzymes to create metabolite deficiencies and suppress tumor growth remains largely underexplored.
α-ketoglutarate (α-KG) (also known as 2-oxoglutarate) dietary supplementation has previously been shown to improve muscle growth and wound healing in addition to demonstrating antiaging effects in nematodes and mice,4 however its role in suppressing tumor growth is underexplored. α-KG is a metabolic intermediate of the Krebs cycle, formed by oxidative decarboxylation of isocitrate, by isocitrate dehydrogenase (IDH). α-KG is an important source of carbon and nitrogen and also a nitrogen scavenger, thereby supporting various aminotransferase reactions that can result in the deamination and catabolism of amino acids. α-KG is also a cosubstrate of the α-KG–dependent dioxygenases (α-KGDDs) that play a central role in regulating cell fate.5 D-2-hydroxyglutarate an oncometabolite elevated in IDH1/2 mutant cancers competitively inhibits α-KGDDs to promote oncogenesis of IDH-mutant cancers. In this study, the authors use this premise to test whether supplementation of α-KG suppresses oncogenesis. Using human diffuse large B-cell lymphoma (DLBCL) cell lines, patient samples and Myc-driven murine B cells, they show permeant supplementation of αKG (DMαKG) to lymphoma cells increases cell death, importantly, regardless of DLBCL cell of origin or genetic characteristics. Normal murine splenic lymphocytes, untransformed human tonsil–isolated B cells and myeloid leukemias were insensitive to DMαKG supplementation. These observations support further investigation into the basis of cell context–specific, α-KG supplementation–induced cell death.
In exploring the effects of α-KG on Wnt/β-catenin signaling the authors show, as previously reported,6 a reduction of transcriptional targets of Wnt/transcription factor 7 binding, induction of TET2-dependent DKK4 expression and a decrease in β-catenin expression. Expression of constitutively active β-catenin, however, did not rescue the effects of DMαKG on lymphoma-cell viability, suggesting other modes of action of α-KG-induced cell death. The authors also ruled out the effects of α-KGDDs, RNA demethylases, and hypoxia-inducible factor prolyl hydroxylases in the tumor suppressive effects of α-KG.
Steady state–targeted metabolomics in DMαKG-supplemented lymphoma lines revealed a significant and sustained decrease in aspartate, alanine, and branched-chain amino acids (BCAAs; leucine, valine, and isoleucine), accompanied by an increase in glutamate and glutamine. These changes are in line with α-KG being used by transaminases like glutamic-oxaloacetic transaminase 1 to catalyze transamination between aspartate and α-KG to form oxaloacetate and glutamate. In addition, the authors demonstrate that branched-chain amino acid transaminase 2 (BCAT2) is a major contributor to the α-KG supplementation–mediated depletion of BCAAs. BCAT2 catalyzes a reversible transamination reaction, where it transfers the amino group from BCAAs to α-KG. Amino acids play a critical role in cancer cells and upregulation of amino acid transporters and elevation of pathways leading to amino acid synthesis is common.
Targeting amino acid uptake and or synthesis to impact cancer cell growth/viability however, has been challenging, thus making strategies like α-KG supplementation an appealing approach. Proliferating cells in general exhibit an increased demand for amino acids. Future studies should examine whether the cytotoxic/growth inhibitory effects of α-KG are dependent upon cell proliferation.
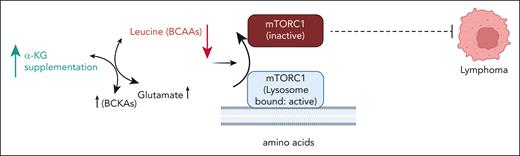
mTORC1 integrates nutrient availability and growth-factor signaling to cell growth, metabolism, and autophagy. Nutrient starvation promotes the release of mTORC1 from the lysosomal surface to the cytoplasm, suppressing its activity.7,8 Leucine, glutamine, and arginine are key regulators of mTORC1 activation. Upon amino acid withdrawal, mTORC1 can be found diffusely throughout the cytoplasm, whereas replenishment of amino acids translocates mTORC1 to the surface of vesicles positive for Rab7 and LAMP2 (lysosome-associated membrane protein 2).9 The authors interestingly showed that excess α-KG creates a leucine-deprived state, reducing mTORC1 lysosomal localization (see figure). α-KG–induced cytotoxicity was rescued by expression of the Raptor-Rheb15 construct rendering mTORC1 lysosomal localization less sensitive to a reduction of intracellular leucine levels. Although the authors demonstrate a reduction of both pP70S6K and p4EBP expression with α-KG supplementation, it will be important for future studies to investigate how α-KG–suppressed mTORC1 signaling leads to cell death. A dietary intervention trial showed increased survival of Eμ-Myc mice, a strain that displays full lymphoma penetrance. Interestingly, mice at early treatment time points demonstrated weight reduction. Future clinical trials are thus needed to determine optimal dosages and long-term effects of α-KG supplementation in humans. Identifying rational combination strategies with α-KG and the effects of combining α-KG with agents targeting proliferation such as DNA-damaging alkylating agents (standard in lymphoma therapy), will be important. Overall, these studies provide further impetus to investigate how metabolic pathways can be hijacked by metabolite supplementation and implemented for cancer therapy.
α-KG supplementation rewires BCAA metabolism to deplete amino acids like leucine. Amino acid deprivation inactivates mTORC1 by perturbing lysosomal localization that contributes to suppression of lymphoma growth. The model does not reflect actual subcellular localization of metabolites. BCAA, branched-chain amino acid; BCKA, branched-chain keto acids. The figure was created using BioRender.com.
α-KG supplementation rewires BCAA metabolism to deplete amino acids like leucine. Amino acid deprivation inactivates mTORC1 by perturbing lysosomal localization that contributes to suppression of lymphoma growth. The model does not reflect actual subcellular localization of metabolites. BCAA, branched-chain amino acid; BCKA, branched-chain keto acids. The figure was created using BioRender.com.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal