Therapeutic targeting of Bruton tyrosine kinase (BTK), a key component of B-cell receptor signaling has transformed care in patients with chronic lymphocytic leukemia (CLL). With the increasing number of BTK inhibitors (BTKi) currently under investigation for CLL treatment, questions remain regarding the sequence and durations of treatment with the different BTKi. In this issue of Blood, Brown et al1 present a pioneering study on genetic changes associated with pirtobrutinib resistance vs those associated with pirtobrutinib response in heavily-pretreated patients.
As a next-generation, highly selective, and noncovalent BTKi (ncBTKi), pirtobrutinib does not depend on cysteine 481 (C481), which is critical for the binding of covalent BTKi (cBTKi) such as ibrutinib, acalabrutinib, and zanubrutinib.2 Hence, pirtobrutinib was considered mainly for treatment of patients who had progressive disease (PD) during treatment with cBTKi. The BRUIN 1/2 trial (NCT03740529) was a multicenter phase 1/2 clinical study evaluating pirtobrutinib in patients with relapsed/refractory (R/R) CLL and small lymphocytic lymphoma. Here, the authors analyzed CLL evolution under pirtobrutinib treatment especially in patients with prior cBTKi treatment, with or without an additional BCL2 inhibitor (BCL2i). The median number of prior therapy lines was 4, and most patients received ibrutinib whereas others received acalabrutinib or zanubrutinib. Targeted next-generation sequencing (tNGS) identified BTK mutations in nearly half of the patient samples prior to pirtobrutinib treatment, predominantly in the C481 locus, however, mutations in L528 and T474 positions were identified but at low frequencies. Recurrent mutations were also identified at baseline in TP53 and PLCG2, among other genes.
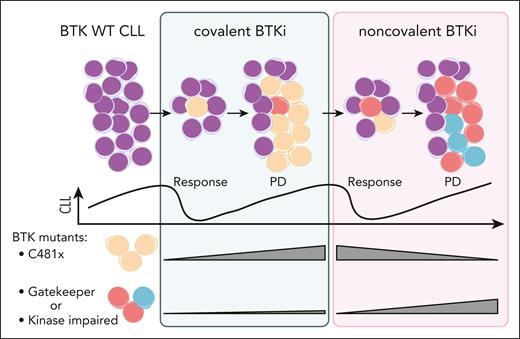
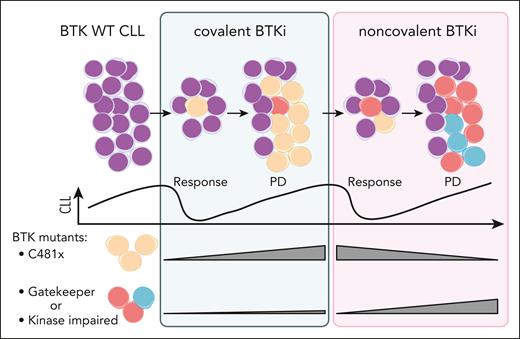
To address mechanisms underlying resistance to pirtobrutinib, Brown et al analyzed samples from a subset of 88 patients with available PD samples in addition to baseline. Notably, 119 mutations identified at baseline were cleared with pirtobrutinib treatment. In particular, BTK mutations in the C481 position were decreased or absent in majority of the patients. Conversely, in samples from PD while receiving pirtobrutinib, 138 new mutations were acquired. About half of the patients gained a BTK mutation, mostly in residues other than C481. These results indicate a clonal shift with change of treatment from cBTKi to ncBTKi (see figure). Resistance patterns emerging at PD under pirtobrutinib reveal both the adaptive capacity of CLL cells and potential limitations of the ncBTKi-based approach. The emergence of gatekeeper T474 mutations and kinase-impaired mutations in L528, D539 poses a challenge for further treatments with both cBTKi and ncBTKi. Although some non-BTK mutations were also acquired at PD in TP53 and PIK3CA, their mechanistic relationship with pirtobrutinib resistance require further investigation.
Changes in BTK clonal architecture under treatment with cBTKi followed by ncBTKi. CLL cells predominantly become resistant to cBTKi by acquiring mutations in C481 of BTK (yellow) that abrogate drug binding. ncBTKi can eliminate the C481 mutant clones. However, in patient subsets, minor subclones in the T474 gatekeeper residue or mutations affecting BTK function (red or blue) can occur under cBTKi. These non-C481 mutations can be clonally selected or newly acquired during ncBTKi treatment, leading to PD. WT, wild-type. Figure created with biorender.com. Jebaraj B. (2025) https://biorender.com/wj8ct2m.
Changes in BTK clonal architecture under treatment with cBTKi followed by ncBTKi. CLL cells predominantly become resistant to cBTKi by acquiring mutations in C481 of BTK (yellow) that abrogate drug binding. ncBTKi can eliminate the C481 mutant clones. However, in patient subsets, minor subclones in the T474 gatekeeper residue or mutations affecting BTK function (red or blue) can occur under cBTKi. These non-C481 mutations can be clonally selected or newly acquired during ncBTKi treatment, leading to PD. WT, wild-type. Figure created with biorender.com. Jebaraj B. (2025) https://biorender.com/wj8ct2m.
One concern from the results is that one-third of the patients with PD did not acquire a mutation in BTK, PLCG2, TP53, or other genes covered by the tNGS panel. This observation highlights a significant gap in our understanding of BTKi resistance mechanisms. Additional studies are needed to delineate signaling pathway changes or novel molecular alterations that confer resistance to pirtobrutinib in the absence of BTK or PLCG2 mutations. This knowledge gap limits our ability to identify patients that are most likely to benefit from pirtobrutinib or alternative therapeutic approaches.
Earlier and current studies have shown that non-C481 mutations, in particular mutations in L5283 and T4744 could also arise under cBTKi treatment. Importantly, using a more sensitive sequencing assay, the authors evaluated prior samples from 79 patients to determine if the mutations designated as newly acquired under pirtobrutinib were already present at very low variant allele frequencies (VAF) before pirtobrutinib. Of interest, 37% of the BTK mutations that were observed at PD with pirtobrutinib treatment were identified at low VAF in the baseline sample. These results indicate that clonal diversification of BTK can occur during first-line BTKi treatments and undergo selection, depending on the type of subsequent BTKi treatment. In contrast, longitudinal NGS analysis of samples from 16 patients who responded long-term to pirtobrutinib had no emergence of novel BTK mutations in the intermediate time points. Thus, cases with the longest durations of response apparently had a lower capability to clonally evolve. Therefore, screening upon PD on cBTKi using a high sensitivity technique such as digital droplet polymerase chain reaction at the L528 and T424 loci may help in the clinically relevant decision of next-line treatment with ncBTKi or alternative approaches. In this context, recent work suggested that proteolysis targeting chimera–based BTK “degraders” (BTKd) may be efficacious in a wide range of BTK mutants.5 However, BTK mutations in A428 have been found to confer resistance also to BTKd,6 highlighting the clinically relevant interplay between biological and therapeutic evolution.
Although the ability to clear C481 mutations supports using pirtobrutinib after cBTKi, no significant difference in overall response rate was observed in cases with and without BTK mutation clearance. The question of whether pirtobrutinib might be more effective when used earlier in CLL before the occurrence of extensive clonal evolution deserves consideration. Conceptionally, the shifting clonal architecture and emergence of resistance across different BTKi classes suggests that continuous single-agent BTK inhibition may have inherent limitations whereas a time-limited or MRD-guided combination treatment may be important for keeping tumor evolution under check.
In conclusion, pirtobrutinib shows encouraging activity in cBTKi-resistant CLL, with the ability to eliminate BTK C481 mutant clones. However, the emergence of novel BTK mutations and the proportion of patients with unexplained mechanisms of refractoriness highlight ongoing challenges. Although these results support the role of pirtobrutinib in targeting R/R CLL, they also underscore the need for more innovative approaches to achieve durable disease control. Future studies are warranted to identify truly predictive biomarkers, to optimize combinations and sequences in the growing CLL treatment armamentarium.
Conflict-of-interest disclosure: S.S. reports receiving personal fees, research support, and nonfinancial support from AbbVie, AstraZeneca, BeOne, Bristol Myers Squibb, Galapagos, F. Hoffmann La-Roche, Johnson & Johnson, Lilly, and Novartis outside of the submitted work. B.M.C.J. declares no competing financial interests.