In this issue of Blood, Meermeier et al1 demonstrate in an immunocompetent murine model (Vk∗MYChCRBN) of multiple myeloma (MM) that pre-treatment with cereblon E3 ligase modulatory drugs (CELMoDs) reshapes the bone marrow (BM) T-cell compartment and overcomes primary resistance to anti–B-cell maturation antigen T-cell engagers (BCMA-TCE).
BCMA-targeted therapies including chimeric antigen receptor (CAR) T-cell therapies and TCE have demonstrated remarkably efficacy in heavily pretreated MM patients. However, responses to these therapies are not universal and acquired resistance occurs often predominantly due to tumor-intrinsic factors such as antigen loss/mutations2,3 and less commonly due to T-cell dysfunction or an immunosuppressive BM microenvironment.4 Primary resistance, defined by the lack of response to initial BCMA-targeted therapies, is seen in 30% of patients5 and caused by high disease burden (stage III of Revised International Staging System disease, >60% BM plasma cells), extramedullary disease or driven by an enrichment of TOX+ exhausted CD8+ cells and a lower proportion of CD8+ CX3CR1 effector cells in the BM, prior to therapy initiation.6 High baseline levels of soluble BCMA (sBCMA) were also recently identified as an independent predictive factor associated with primary refractoriness to anti–BCMA-TCE.7 sBCMA can directly inhibits TCE binding to MM cells and together with surface BCMA-antigen density influences TCE-cytolytic activity. This effect can be partially abrogated by increased effector-to-target (E:T) ratio. The E:T ratio, representing the ratio of effector (T cells) to target (MM cells), is an important factor for TCE activity. TCE are designed to engage T cells via the CD3ϵ subunit of the T-cell receptor complex and bring them in proximity of tumor cells enabling the formation of immunological synapses and subsequent tumor cell death.8 Therefore, a higher E:T ratio can lead to improved T-cell engagement and increased treatment efficacy to overcome resistance. Although increasing TCE concentration may not always be sufficient, particularly if lymphocytes counts are reduced.7
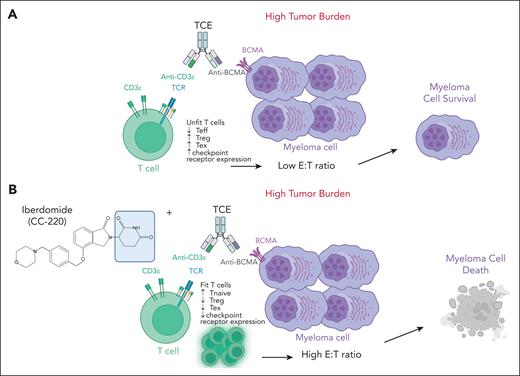
In the study by Meermeier et al, the authors used an immunocompetent preclinical model of MM, the Vk∗MYChCRBN, that faithfully mimics MM tumor genomic complexity to evaluate high tumor burden strategies to improve anti–BCMA-TCE response, while maintaining a manageable safety profile. Of interest, they show that the triple combination of BCMA-TCE, pomalidomide, and PD1 blockade was able to reduce the T-cell exhaustion that occurs in high disease burden following TCE treatment, leading to sustained remission in the treated mice. However, the induced persistent activation of cytolytic T cells led to lethal cytokine release syndrome (CRS) in a significant subset of mice. To maintain efficacy but prevent fatal CRS they tested a step-up dose of BCMA-TCE and a pretreatment with the CELMoD iberdomide and dexamethasone and demonstrated complete response and long survival in all mice with high tumor burden. CELMoDs, including iberdomide and mezigdomide, are a new class of agents known for their anti-MM activity and ability to promote the innate and adaptive immune responses.9 Meermeier et al longitudinally tracked the dynamic changes in T-cell activity occurring during TCE therapy and demonstrated the ability of CELMoDs to induce a favorable T-cell profile with increased number of naïve T cells, limited expansion of regulatory T cells and reduced T-cell exhaustion (see figure). Such reshaping of the BM T-cell compartment was associated with T-cell proliferation and infiltration near the sites of tumor resulting in an increased E:T ratio necessary to overcome primary resistance to TCE.
Shown here is the ability of CELMoDs to reshape the BM T-cell compartment and increase the E:T ratio to enhance anti–BCMA-TCE efficacy in high disease burden. (A) Lack of activity of anti–BCMA-TCE when used alone in high disease burden. (B) Ability of CELMoDs to promote T-cell proliferation increasing the E:T ratio and overcoming primary resistance in high disease burden. The figure was created with BioRender.com. Neri P. (2025) https://app.biorender.com/illustrations/68900265a0a2a697614a88ec. TCR, T-cell receptor; Teff, T-effectors; Tex, T-exhausted; Treg, T-regulatory cells.
Shown here is the ability of CELMoDs to reshape the BM T-cell compartment and increase the E:T ratio to enhance anti–BCMA-TCE efficacy in high disease burden. (A) Lack of activity of anti–BCMA-TCE when used alone in high disease burden. (B) Ability of CELMoDs to promote T-cell proliferation increasing the E:T ratio and overcoming primary resistance in high disease burden. The figure was created with BioRender.com. Neri P. (2025) https://app.biorender.com/illustrations/68900265a0a2a697614a88ec. TCR, T-cell receptor; Teff, T-effectors; Tex, T-exhausted; Treg, T-regulatory cells.
These findings have great clinical significance because several strategies are currently under evaluation to overcome resistance to BCMA-targeted therapies. This includes combination approaches with γ-secretase inhibitors to prevent BCMA cleavage and shedding and increase BCMA surface expression. This combination has been examined in several studies and has demonstrated encouraging results in early clinical data.10 Furthermore, in patients with a high disease burden, strategies aimed at overcoming T-cell dysfunction, and the BM immune suppressive microenvironment are currently under evaluation. The combination of TCE with immune checkpoint inhibitors appears of interest due to their ability to enhance T-cell activation. However, this approach requires caution due to severe toxicity as previously reported and confirmed in this study.1 Better strategies aimed at enhancing the E:T ratio include the use of immunomodulatory drugs (IMiDs) or CELMoDs to restore T-cell function while enhancing anti-MM activity, as nicely demonstrated in this study.1 Similarly, combinations of BCMA-TCE with other anti-MM drugs such as anti-CD38 antibodies to increase disease debulking while depleting CD38 regulatory T and B cells have also the potential of creating a favorable E:T ratio to overcome primary resistance and are at present under evaluation.
As these therapies move to earlier lines in the disease course, including the frontline and maintenance setting, it is critical to understand the dynamic changes in the T-cell activity and immune BM microenvironment that occur during TCE treatment. This information can indeed guide the design of strategies able to induce responses in patients with high disease burden, as well as in extramedullary disease, yielding more durable responses and evading the development of resistance.
Consequently, the work presented in this study provides solid rational to use CELMoDs to reshape the BM milieu by expanding the T-cell compartment, “outnumbering” MM cells to produce favorable E:T ratio and enhance TCE efficacy when given concurrently or as a pretreatment.1 The clinical evaluation of such strategies is currently ongoing and will provide further insight into the clinical relevance of this approach.
Conflicts-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal