Key Points
Transfer of Cd47−/− RBCs to WT mice activates cDCs and promotes retention and macrophage-mediated phagocytosis of the RBCs in the spleen.
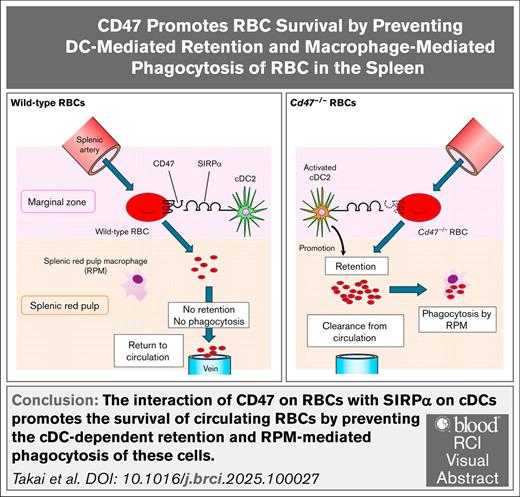
Interaction of CD47 on circulating RBCs with SIRPα on cDCs promotes RBC survival by preventing their cDC-mediated retention in the spleen.
Visual Abstract
The interaction of CD47 on red blood cells (RBCs) with signal regulatory protein α (SIRPα) on macrophages prevents phagocytosis of RBCs by macrophages. The transfusion of CD47-deficient (Cd47−/−) RBCs into wild-type (WT) mice indeed results in marked phagocytosis of these cells by splenic red pulp macrophages (RPMs) and their consequent rapid elimination from the recipients. This study confirmed that transfusion of Cd47−/− RBCs resulted in the activation of conventional dendritic cells (cDCs) in the spleen of WT recipient mice as previously reported. In addition, clearance of the transferred Cd47−/− RBCs was found to be prevented by depletion of both cDCs and RPMs but only partially attenuated by that of RPMs alone. The transfusion of Cd47−/− RBCs into WT recipients was also found to result in marked retention of the RBCs in the spleen before their phagocytosis by RPMs. Such retention was not affected by depletion of RPMs alone but was greatly attenuated by depletion of both cDCs and RPMs. SIRPα-expressing CD4+ cDC2s appeared to contribute to the retention and rapid clearance of transfused Cd47−/− RBCs. Collectively, the findings indicate that the interaction of CD47 on RBCs with SIRPα on cDCs promotes the survival of circulating RBCs by preventing the cDC-dependent retention and RPM-mediated phagocytosis of these cells.
Introduction
The clearance of senescent or abnormal red blood cells (RBCs) by the spleen is achieved mostly as a result of their specific recognition and phagocytosis by splenic red pulp macrophages (RPMs).1 However, the precise molecular mechanism by which RPMs or other innate immune cells, such as dendritic cells (DCs), recognize senescent RBCs and thereby mediate their elimination has remained unknown.
Expression of CD47, an immunoglobulin superfamily penta-transmembrane protein,2 on the RBC surface promotes the survival of healthy cells by preventing their elimination by RPMs.3 CD47 binds through its extracellular region to another immunoglobulin superfamily transmembrane protein, signal regulatory protein α (SIRPα), which is prominently expressed on macrophages. Such interaction of CD47 with SIRPα is thought to negatively regulate the phagocytosis by macrophages.3 CD47, thus, prevents phagocytosis of RBCs by RPMs and, thereby, promotes the survival of healthy RBCs.4
Previously, it was found that, in contrast to the rapid elimination of transfused CD47-deficient (Cd47−/−) RBCs from the bloodstream of wild-type (WT) recipient mice,4 the transfusion of WT RBCs into SIRPα-deficient mice did not result in the immediate elimination of these cells, whereas Ig-opsonized WT RBCs transfused into SIRPα-deficient mice were eliminated more rapidly compared with those transfused into WT mice.5 Moreover, the phagocytosis of immunoglobulin-opsonized WT RBCs by SIRPα-deficient RPMs in vitro was markedly enhanced relative to that mediated by WT RPMs, whereas such enhancement was not apparent for nonopsonized RBCs.5 Collectively, these findings suggest that the binding of CD47 on RBCs to SIRPα on RPMs prevents the Fcγ receptor–mediated phagocytosis of immunoglobulin-opsonized RBCs by RPMs. In contrast, the rapid elimination of nonopsonized Cd47−/− RBCs from WT recipient mice likely reflects a CD47-dependent mechanism to prevent the elimination of healthy RBCs by RPMs or other immune cells.
Transfusion of Cd47−/− RBCs into WT mice was also recently found to result in the activation of CD11c+ conventional DCs (cDCs), which also express SIRPα,6,7 in the spleen of the recipient mice.8,9 Therefore, it is evaluated whether such activation of cDCs might participate in the rapid elimination of transfused Cd47−/− RBCs from the bloodstream of WT recipient mice to clarify whether CD47 on RBCs, through its interaction with SIRPα on cDCs, promotes the survival of circulating RBCs in vivo.
Methods
Animals
C57BL/6J mice were from Japan SLC (Shizuoka, Japan). Systemic Cd47−/− mice were generated as described previously.10 For generation of CD11ciDTRΔ mice (with DC-specific expression of the diphtheria toxin receptor [DTR]), CD11c-iDTR mice11 were crossed with E2A-Cre mice, which were from The Jackson Laboratory (Bar Harbor, ME). XCR1DTRvenus mice12,13 and Sirpaf/f and Itgax-Cre;Sirpaf/f (SirpaΔDC) mice7 were generated as described previously.
In vivo clearance of RBCs
In vivo clearance of RBCs was determined as described previously,5,14,15 with minor modifications. In brief, peripheral blood (100 μL) from WT or systemic Cd47−/− mice was incubated with 1.5 μM carboxyfluorescein succinimidyl ester (CFSE; Sigma-Aldrich) or 5 μM CytoRed (Fujifilm Wako, Osaka, Japan) for 20 minutes at 37°C, and the cells were washed 3 times with and then suspended (usually at a density of 1 × 109 cells per 100 μL) in sterile phosphate-buffered saline (PBS). Recipient mice were injected IV with the labeled RBCs, and peripheral blood samples (5 μL) were collected from the tail vein at the indicated times. The fraction of labeled RBCs among all RBCs (total of 10 000 cells) was determined by flow cytometry with an LSR Fortessa X-20 (BD Biosciences) or MACSQuanto (Miltenyi Biotec, Bergisch Gladbach, Germany) instrument. Data were analyzed with FlowJo X software (BD Biosciences).
Cell preparation and flow cytometry
For splenic cell analysis, cell suspensions were prepared from the spleen as described previously.16 In brief, tissue was minced with scissors and then digested for 20 minutes at 37°C in RPMI 1640 medium (Fujifilm Wako) containing collagenase IV (Worthington Biochemical, Lakewood, NJ) at 1 mg/mL and DNase I (Sigma-Aldrich) at 40 μg/mL. Undigested fibrous material was removed by filtration through a 70 μm nylon mesh. RBCs in the filtrate were lysed with ammonium-chloride-potassium buffer (150 mM NH4Cl, 10 mM KHCO3), and the remaining cells were washed and suspended in fluorescence-activated cell sorting (FACS) buffer (PBS supplemented with 2% fetal bovine serum and 2 mM EDTA). For peripheral blood cell analysis, 100 μL of blood was collected from tail vein and washed with FACS buffer. RBCs were lysed using ammonium-chloride-potassium buffer twice, and the remaining cells were then washed and resuspended in FACS buffer. They were processed with a Zombie Aqua Fixable Viability Kit (BioLegend) for discrimination between live and dead cells and were incubated with specific antibodies for 20 minutes at 4°C before flow cytometric analysis performed with an LSR Fortessa X-20 or MACSQuanto instrument. Data were analyzed with FlowJo X software.
Depletion of RPMs and DCs by CL treatment
For depletion of RPMs, 50 μL of clodronate liposomes (CLs; Liposoma BV, Amsterdam, The Netherlands) were injected IV into WT mice on day 0 and the mice were analyzed on day 7. For depletion of RPMs and DCs, 100 μL of CLs were injected IV into WT mice on day 0 and day 1 and the mice were analyzed on day 2. The same volume of PBS liposomes as CLs was administered to control mice.
Depletion of DCs by DTx treatment
For depletion of CD11c-positive DCs, 1 μg of diphtheria toxin (DTx, Sigma-Aldrich) was injected intraperitoneally into CD11ciDTRΔ mice on day 0 and the mice were analyzed on day 2. For depletion of X-C motif chemokine receptor 1 (XCR1)–positive cDC1s, 0.8 μg of DTx was injected intraperitoneally into XCR1DTRvenus mice on day 0 and the mice were analyzed on day 1.
Analysis of RBC retention in the spleen
CFSE- or CytoRed-labeled peripheral blood cells were injected IV into recipient mice as described previously. After 2 hours, the spleen was removed from the recipient animals, and the tissue was minced and digested for 20 minutes at 37°C with RPMI 1640 medium containing collagenase IV at 1 mg/mL, DNase I at 40 μg/mL, and 2% fetal bovine serum. The undigested material was removed by filtration through a 70 μm nylon mesh, and the isolated cells were washed and resuspended in FACS buffer, processed with a Zombie Aqua Fixable Viability Kit (BioLegend), incubated with specific antibodies for 20 minutes at 4°C, and subjected to flow cytometry with an LSR Fortessa X-20 or MACSQuanto instrument. Data were analyzed with FlowJo X software.
Antibodies, regents, immunohistofluorescence analysis, transmission electron microscopy (TEM), and statistical analysis can be found in the supplemental Methods.
All animals were maintained at the Institute for Experimental Animals, Kobe University Graduate School of Medicine, under specific pathogen-free conditions. All animal experiments were performed according to the guidelines of and were approved by the Animal Care and Experimentation Committee of Kobe University (permit numbers: P201009R4 and P240604).
Results
Transfusion of Cd47−/− RBCs into WT mice induces cDC activation and incorporation into RPMs and clearance of the transferred RBCs
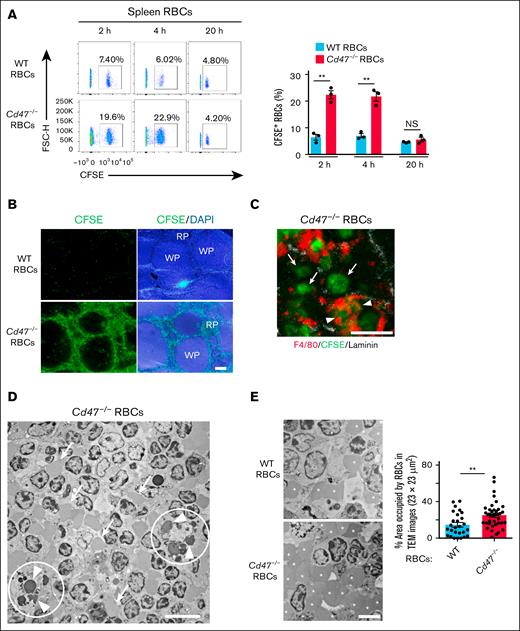
Consistent with previous observations,4,14 the transfusion of Cd47−/− RBCs into WT mice resulted in the rapid elimination of these cells from the peripheral blood (Figure 1A). The incorporation of transferred Cd47−/− RBCs into RPMs of WT recipients was also markedly increased (Figure 1B; supplemental Figure 1A). In addition, transfusion of Cd47−/− RBCs into WT mice was previously found to induce activation of CD11c+ cDCs in the spleen.8,9 Indeed, the transfusion of Cd47−/− RBCs, but not that of WT RBCs, was confirmed to result in a marked increase in the expression of activation markers for cDCs, including CD86, CCR7, and major histocompatibility complex class II (MHCII), in CD8+ cDC1s and CD4+ cDC2s (Figure 1C-E; supplemental Figure 1B). These results suggested that the transfusion of Cd47−/− RBCs indeed induced a rapid activation of cDCs in parallel with incorporation of the transfused cells into RPMs and their elimination from the bloodstream of WT recipient mice.
Transfusion of Cd47−/− RBCs into WT mice induces cDC activation in parallel with incorporation of the transferred cells into RPMs and their clearance from the bloodstream. (A) Clearance of WT or Cd47−/− RBCs from peripheral blood of WT recipient mice. Data are means ± standard error of the mean (SEM) for 3 recipient mice. (B) Representative flow cytometric plots (left panel) and the percentage of CFSE-positive RPMs among total RPMs (right panel) for WT recipient mice at 2 hours after IV injection of CFSE-labeled WT or Cd47−/− RBCs. Data for mice that were not injected with RBCs are also revealed (RBC iv[−]). Data in the right panel are means ± SEM for 3 recipient mice. (C-E) Representative flow cytometric analysis of CD86 (C), CCR7 (D), and MHCII (E) expression (left panels) and quantitative data for MFI (right panels) for cDC subsets of WT recipient mice at 2 hours after IV injection of CFSE-labeled WT or Cd47−/− RBCs. Data for mice that were not injected with RBCs are also revealed (RBC iv[−]). Data in the right panels are means ± SEM for 3 recipient mice. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 by 2-way repeated-measures analysis of variance (ANOVA) with Tukey’s multiple-comparison test (A) or by 1-way ANOVA with Tukey’s multiple-comparison test (B-E). MFI, mean fluorescence intensity.
Transfusion of Cd47−/− RBCs into WT mice induces cDC activation in parallel with incorporation of the transferred cells into RPMs and their clearance from the bloodstream. (A) Clearance of WT or Cd47−/− RBCs from peripheral blood of WT recipient mice. Data are means ± standard error of the mean (SEM) for 3 recipient mice. (B) Representative flow cytometric plots (left panel) and the percentage of CFSE-positive RPMs among total RPMs (right panel) for WT recipient mice at 2 hours after IV injection of CFSE-labeled WT or Cd47−/− RBCs. Data for mice that were not injected with RBCs are also revealed (RBC iv[−]). Data in the right panel are means ± SEM for 3 recipient mice. (C-E) Representative flow cytometric analysis of CD86 (C), CCR7 (D), and MHCII (E) expression (left panels) and quantitative data for MFI (right panels) for cDC subsets of WT recipient mice at 2 hours after IV injection of CFSE-labeled WT or Cd47−/− RBCs. Data for mice that were not injected with RBCs are also revealed (RBC iv[−]). Data in the right panels are means ± SEM for 3 recipient mice. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 by 2-way repeated-measures analysis of variance (ANOVA) with Tukey’s multiple-comparison test (A) or by 1-way ANOVA with Tukey’s multiple-comparison test (B-E). MFI, mean fluorescence intensity.
Two injections of CLs reduce RPMs, cDCs, and monocytes and abolish the rapid elimination of transfused Cd47−/− RBCs from WT recipient mice
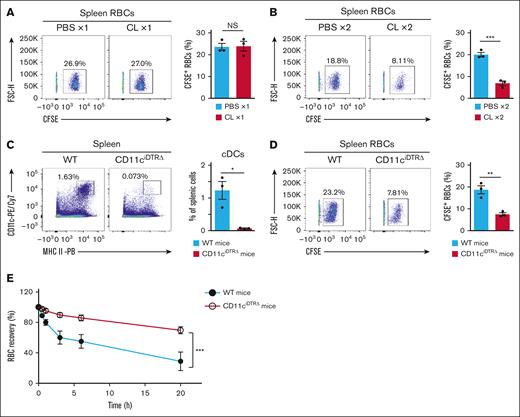
The rapid elimination of transfused Cd47−/− RBCs from WT recipient mice was previously found to be markedly attenuated by prior treatment of the mice with CLs to deplete RPMs.4 In this study, the effect of such CL treatment on the population of RPMs and on that of splenic cDCs was evaluated. A single injection of 50 μL CLs indeed resulted in marked depletion of RPMs, but it failed to affect the population of splenic cDCs (Figure 2A; supplemental Figure 2A). By contrast, two injections of 100 μL CLs induced depletion of splenic cDCs and RPMs (Figure 2B; supplemental Figure 2B). Furthermore, both a single injection and 2 injections of CLs were found to increase neutrophils in the spleen (supplemental Figure 3A,C,E). Although these treatments tended to increase the number of neutrophils in the peripheral blood, the increase was not statistically significant (supplemental Figure 3B,D,F). In addition, a single injection of CLs did not affect the number of monocytes, whereas 2 injections of CLs decreased these cells in the spleen and peripheral blood (supplemental Figure 3). The depletion of RPMs alone by CLs only partly attenuated the rapid elimination of transfused Cd47−/− RBCs (Figure 2C). In contrast, 2 injections of CLs abolished the rapid clearance of the transfused cells (Figure 2D). Given that 2 injections of, but not a single injection of, CLs decreased cDCs and monocytes in the spleen, these phagocytes in addition to RPMs are likely important for the rapid elimination of transfused Cd47−/− RBCs from WT recipient mice.
Two injections of CLs abolish the rapid elimination of transfused Cd47−/− RBCs from WT recipient mice. (A) Representative flow cytometric plots of RPMs (upper left panel) and cDCs (lower left panel) and the percentage of RPMs among total splenic cells (upper right panel) and that of cDCs among total splenic cells (lower right panel) on day 7 after treatment of mice with 50 μL of CLs (CL ×1) or PBS liposomes (PBS ×1) on day 0. Data in the right panels are means ± SEM for 4 mice. (B) Representative flow cytometric plots of RPMs (upper left panel) and cDCs (lower left panel) and the percentage of RPMs among total splenic cells (upper right panel) and that of cDCs among total splenic cells (lower right panel) on day 2 after treatment of mice with 100 μL of CLs (CL ×2) or PBS liposomes (PBS ×2) on day 0 and day 1. Data in the right panels are means ± SEM for 3 mice. (C) Clearance of transfused Cd47−/− RBCs from peripheral blood of WT recipient mice treated with CLs (CL ×1) or PBS liposomes (PBS ×1) as in panel A. Data for WT recipient mice injected with WT RBCs but not treated with liposomes (WT RBCs [no treatment]) are also revealed. The data in the lower panel are a more detailed representation of those in the upper panel. All data are means ± SEM for 3 recipient mice. (D) Clearance of transfused Cd47−/− RBCs from the peripheral blood of WT recipient mice treated with CLs (CL ×2) or PBS liposomes (PBS ×2) as in panel B. Data for WT recipient mice injected with WT RBCs but not treated with liposomes (WT RBCs [no treatment]) are also revealed. The data in the lower panel are a more detailed representation of those in the upper panel. All data are means ± SEM for 3 recipient mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 by Student t test (A-B) or by 2-way repeated-measures ANOVA with Tukey’s multiple-comparison test (C-D). NS, not significant.
Two injections of CLs abolish the rapid elimination of transfused Cd47−/− RBCs from WT recipient mice. (A) Representative flow cytometric plots of RPMs (upper left panel) and cDCs (lower left panel) and the percentage of RPMs among total splenic cells (upper right panel) and that of cDCs among total splenic cells (lower right panel) on day 7 after treatment of mice with 50 μL of CLs (CL ×1) or PBS liposomes (PBS ×1) on day 0. Data in the right panels are means ± SEM for 4 mice. (B) Representative flow cytometric plots of RPMs (upper left panel) and cDCs (lower left panel) and the percentage of RPMs among total splenic cells (upper right panel) and that of cDCs among total splenic cells (lower right panel) on day 2 after treatment of mice with 100 μL of CLs (CL ×2) or PBS liposomes (PBS ×2) on day 0 and day 1. Data in the right panels are means ± SEM for 3 mice. (C) Clearance of transfused Cd47−/− RBCs from peripheral blood of WT recipient mice treated with CLs (CL ×1) or PBS liposomes (PBS ×1) as in panel A. Data for WT recipient mice injected with WT RBCs but not treated with liposomes (WT RBCs [no treatment]) are also revealed. The data in the lower panel are a more detailed representation of those in the upper panel. All data are means ± SEM for 3 recipient mice. (D) Clearance of transfused Cd47−/− RBCs from the peripheral blood of WT recipient mice treated with CLs (CL ×2) or PBS liposomes (PBS ×2) as in panel B. Data for WT recipient mice injected with WT RBCs but not treated with liposomes (WT RBCs [no treatment]) are also revealed. The data in the lower panel are a more detailed representation of those in the upper panel. All data are means ± SEM for 3 recipient mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 by Student t test (A-B) or by 2-way repeated-measures ANOVA with Tukey’s multiple-comparison test (C-D). NS, not significant.
Transfusion of Cd47−/− RBCs into WT recipient mice results in retention of the cells in splenic red pulp
Next, the detailed mechanism of the rapid clearance of transfused Cd47−/− RBCs from WT recipient mice was investigated. The transfusion of Cd47−/− RBCs was found to result in a marked change in the color of the recipient mouse spleen from light red to dark red (supplemental Figure 4). Transfusion of Cd47−/− RBCs into WT mice indeed resulted in a large increase in the number of the CFSE-labeled cells (those not incorporated into RPMs) in the spleen at 2 and 4 hours after the transfusion (Figure 3A; supplemental Figure 5). However, Cd47−/− RBCs did not promote the accumulation of endogenous WT RBCs in the spleen at 2 hours after the transfusion (supplemental Figure 6). Transfusion of Cd47−/− RBCs into WT recipient mice thus appeared to result in retention of these cells in the spleen before their phagocytosis.
Transfusion of Cd47−/− RBCs into WT recipient mice results in retention of these cells in RP. (A) Representative flow cytometric plots (left panel) and the percentage (right panel) of CFSE-positive RBCs among total splenic RBCs of WT recipient mice at 2, 4, and 20 hours after IV injection of CFSE-labeled WT or Cd47−/− RBCs. The data in the right panel are means ± SEM for 3 recipient mice. (B) Representative fluorescence microscopic images of frozen sections for the spleen of WT recipient mice at 2 hours after IV injection of CFSE-labeled WT or Cd47−/− RBCs. Fluorescence images for CFSE (green) with or without DAPI (blue) are shown. Scale bar, 200 μm. (C) Representative fluorescence microscopic image of a frozen section for the spleen of a WT recipient mouse at 2 hours after IV injection of CFSE-labeled Cd47−/− RBCs (green). The section was also subjected to immunohistofluorescence analysis with antibodies to F4/80 (red) and to laminin (white). Scale bar, 10 μm. Arrowheads and arrows indicate CFSE-labeled Cd47−/− RBCs that are surrounded or not surrounded by F4/80-positive signals, respectively. (D) Representative TEM image of a spleen section from a WT recipient mouse at 2 hours after IV injection of Cd47−/− RBCs. The white circles include RPMs that appear to be engaged in phagocytosis of RBCs (arrowheads). The arrows indicate RBCs that are localized independently of RPMs. Scale bar, 10 μm. (E) Representative TEM images of RP for WT recipient mice at 2 hours after IV injection of WT (upper left panel) or Cd47−/− (lower left panel) RBCs. Asterisks indicate RBCs positioned independently of RPMs. Scale bar, 5 μm. The percentage area occupied by RBCs in such TEM images (23 × 23 μm2) of RP in WT recipient mice is also revealed (right panel). The data are means ± SEM for 30 TEM images from 3 mice. ∗∗P < .01 by Student t test (panels A,E). DAPI, 4′,6-diamidino-2-phenylindole; FSC-H, forward scatter height; NS, not significant; RP, red pulp; WP, white pulp.
Transfusion of Cd47−/− RBCs into WT recipient mice results in retention of these cells in RP. (A) Representative flow cytometric plots (left panel) and the percentage (right panel) of CFSE-positive RBCs among total splenic RBCs of WT recipient mice at 2, 4, and 20 hours after IV injection of CFSE-labeled WT or Cd47−/− RBCs. The data in the right panel are means ± SEM for 3 recipient mice. (B) Representative fluorescence microscopic images of frozen sections for the spleen of WT recipient mice at 2 hours after IV injection of CFSE-labeled WT or Cd47−/− RBCs. Fluorescence images for CFSE (green) with or without DAPI (blue) are shown. Scale bar, 200 μm. (C) Representative fluorescence microscopic image of a frozen section for the spleen of a WT recipient mouse at 2 hours after IV injection of CFSE-labeled Cd47−/− RBCs (green). The section was also subjected to immunohistofluorescence analysis with antibodies to F4/80 (red) and to laminin (white). Scale bar, 10 μm. Arrowheads and arrows indicate CFSE-labeled Cd47−/− RBCs that are surrounded or not surrounded by F4/80-positive signals, respectively. (D) Representative TEM image of a spleen section from a WT recipient mouse at 2 hours after IV injection of Cd47−/− RBCs. The white circles include RPMs that appear to be engaged in phagocytosis of RBCs (arrowheads). The arrows indicate RBCs that are localized independently of RPMs. Scale bar, 10 μm. (E) Representative TEM images of RP for WT recipient mice at 2 hours after IV injection of WT (upper left panel) or Cd47−/− (lower left panel) RBCs. Asterisks indicate RBCs positioned independently of RPMs. Scale bar, 5 μm. The percentage area occupied by RBCs in such TEM images (23 × 23 μm2) of RP in WT recipient mice is also revealed (right panel). The data are means ± SEM for 30 TEM images from 3 mice. ∗∗P < .01 by Student t test (panels A,E). DAPI, 4′,6-diamidino-2-phenylindole; FSC-H, forward scatter height; NS, not significant; RP, red pulp; WP, white pulp.
Examination of the spleen by fluorescence microscopy also revealed that transfused CFSE-labeled Cd47−/− RBCs accumulated in the splenic red pulp (RP) of WT recipient mice, whereas similarly transfused CFSE-labeled WT RBCs were rarely detected (Figure 3B). Observation at higher magnification revealed that CFSE-labeled Cd47−/− RBCs frequently overlapped with F4/80-positive RPMs (Figure 3C), suggesting that these Cd47−/− RBCs were phagocytosed by RPMs. However, many of the CFSE-labeled Cd47−/− RBCs did not colocalize with RPMs (Figure 3C). TEM also revealed that a proportion of RBCs appeared to be phagocytosed by RPMs, but that many of these cells were also localized independently of RPMs (Figure 3D). In addition, the percentage area occupied by RBCs independently of RPMs in TEM images was markedly increased for RP of WT recipient mice transfused with Cd47−/− RBCs (Figure 3E). These results suggested that transfusion of Cd47−/− RBCs into WT recipient mice resulted in marked retention of the mutant RBCs in RP before their phagocytosis by RPMs.
Importance of cDCs for splenic retention and rapid clearance of transfused Cd47−/− RBCs in WT recipient mice
We next investigated whether RPMs or other phagocytes indeed participate in the splenic retention of transfused Cd47−/− RBCs in WT recipient mice. Whereas treatment of WT mice with a single injection of CLs as in Figure 2A did not prevent the retention of transfused Cd47−/− RBCs in the spleen (Figure 4A), that with 2 injections of CLs as in Figure 2B significantly attenuated the retention of these cells (Figure 4B). Given that cDCs and monocytes in the spleen were markedly depleted by 2 injections but not by a single injection of CLs (Figure 2A-B; supplemental Figure 3C,E), these results implicated cDCs or monocytes in the retention of transfused Cd47−/− RBCs in the spleen of WT recipient mice.
Importance of cDCs for the splenic retention and rapid clearance of Cd47−/− RBCs transfused into WT recipient mice. (A) Representative flow cytometric plots (left panel) and the percentage (right panel) of CFSE-labeled Cd47−/− RBCs among total splenic RBCs at 2 hours after IV injection of the CFSE-labeled cells in WT recipient mice previously subjected to a single injection of CLs (CL ×1) or PBS liposomes (PBS ×1) as in Figure 2A. The data in the right panel are means ± SEM for 3 recipient mice. (B) Representative flow cytometric plots (left panel) and the percentage (right panel) of CFSE-labeled Cd47−/− RBCs among total splenic RBCs at 2 hours after IV injection of the CFSE-labeled cells in WT recipient mice previously subjected to 2 injections of CLs (CL ×2) or PBS liposomes (PBS ×2) as in Figure 2B. The data in the right panel are means ± SEM for 3 recipient mice. (C) Representative flow cytometric plots (left panel) and the percentage (right panel) of cDCs among total splenic cells of WT or CD11ciDTRΔ mice at 2 days after DTx treatment. The data in the right panel are means ± SEM for 3 recipient mice. (D) Representative flow cytometric plots (left panel) and the percentage (right panel) of CFSE-positive Cd47−/− RBCs among total splenic RBCs at 2 hours after IV cell injection in WT or CD11ciDTRΔ mice that had been treated with DTx 2 days previously. The data in the right panel are means ± SEM for 3 recipient mice. (E) Clearance of transfused Cd47−/− RBCs from peripheral blood of WT or CD11ciDTRΔ mice at 2 days after DTx injection. Data are means ± SEM for 3 recipient mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 by Student t test (A-D) or by 2-way repeated-measures ANOVA with Tukey’s multiple-comparison test (E). NS, not significant.
Importance of cDCs for the splenic retention and rapid clearance of Cd47−/− RBCs transfused into WT recipient mice. (A) Representative flow cytometric plots (left panel) and the percentage (right panel) of CFSE-labeled Cd47−/− RBCs among total splenic RBCs at 2 hours after IV injection of the CFSE-labeled cells in WT recipient mice previously subjected to a single injection of CLs (CL ×1) or PBS liposomes (PBS ×1) as in Figure 2A. The data in the right panel are means ± SEM for 3 recipient mice. (B) Representative flow cytometric plots (left panel) and the percentage (right panel) of CFSE-labeled Cd47−/− RBCs among total splenic RBCs at 2 hours after IV injection of the CFSE-labeled cells in WT recipient mice previously subjected to 2 injections of CLs (CL ×2) or PBS liposomes (PBS ×2) as in Figure 2B. The data in the right panel are means ± SEM for 3 recipient mice. (C) Representative flow cytometric plots (left panel) and the percentage (right panel) of cDCs among total splenic cells of WT or CD11ciDTRΔ mice at 2 days after DTx treatment. The data in the right panel are means ± SEM for 3 recipient mice. (D) Representative flow cytometric plots (left panel) and the percentage (right panel) of CFSE-positive Cd47−/− RBCs among total splenic RBCs at 2 hours after IV cell injection in WT or CD11ciDTRΔ mice that had been treated with DTx 2 days previously. The data in the right panel are means ± SEM for 3 recipient mice. (E) Clearance of transfused Cd47−/− RBCs from peripheral blood of WT or CD11ciDTRΔ mice at 2 days after DTx injection. Data are means ± SEM for 3 recipient mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 by Student t test (A-D) or by 2-way repeated-measures ANOVA with Tukey’s multiple-comparison test (E). NS, not significant.
Given that cDCs were activated by the transfusion of Cd47−/− RBCs (Figure 1C-E), investigating the roles of these cells in the rapid clearance of transfused Cd47−/− RBCs was the focus in subsequent experiments. It is confirmed that transfused Cd47−/− RBCs were incorporated into splenic cDCs of WT recipients (supplemental Figure 7), consistent with previous findings.8 However, the ratio of cDCs incorporating Cd47−/− RBCs was lower than that of RPMs incorporating Cd47−/− RBCs (Figure 1B; supplemental Figure 7). Thus, transfused Cd47−/− RBCs are thought to be phagocytosed mainly by RPMs, although some are likely to be incorporated by cDCs in the spleen of WT recipient mice.
To clarify the importance of cDCs for the rapid clearance and the splenic retention of Cd47−/− RBCs transfused into WT recipient mice, CD11ciDTRΔ mice were next studied, in which splenic cDCs are specifically and markedly depleted in response to the administration of DTx.11 Indeed, splenic cDCs were efficiently depleted at 2 days after intraperitoneal injection of DTx in these mice (Figure 4C). CD11b+ cells including monocytes have been demonstrated not to be depleted in DTx-treated CD11ciDTRΔ mice.11 Furthermore, the retention of transferred Cd47−/− RBCs was found to markedly attenuate in DTx-treated CD11ciDTRΔ mice compared with that apparent in DTx-treated WT mice (Figure 4D). Moreover, the clearance of these cells from the peripheral blood was significantly inhibited in the DTx-treated CD11ciDTRΔ mice compared with the DTx-treated WT mice (Figure 4E).11 These results supported the notion that the splenic retention and the rapid clearance of transfused Cd47−/− RBCs in WT recipient mice require cDCs.
Importance of SIRPα+ cDC2s for splenic retention and rapid elimination of Cd47−/− RBCs in WT recipient mice
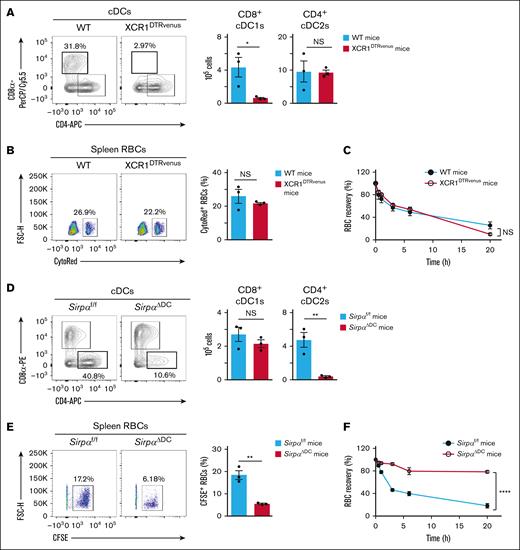
Given that the transfusion of Cd47−/− RBCs in WT recipient mice resulted in the activation of splenic CD8+ cDC1s and CD4+ cDC2s (Figure 1C-E), the latter of which prominently express SIRPα,7 whether CD8+ cDC1s or CD4+ cDC2s indeed participate in the splenic retention and rapid clearance of the transfused Cd47−/− RBCs was evaluated. Treatment of XCR1DTRvenus mice with DTx resulted in marked depletion of CD8+ cDC1s in the spleen, without affecting the splenic population of CD4+ cDC2s (Figure 5A), consistent with previous findings.13 However, splenic retention and rapid clearance of transfused Cd47−/− RBCs in DTx-treated XCR1DTRvenus mice were found to be similar to those apparent for DTx-treated WT mice (Figure 5B-C).
Importance of SIRPα+ cDC2s for the splenic retention and rapid elimination of Cd47−/− RBCs in WT recipient mice. (A) Representative flow cytometric plots for cDC subsets (left panel) and the absolute number of these cells (right panel) in the spleen of WT or XCR1DTRvenus mice at 1 day after DTx treatment. Data in the right panel are means ± SEM for 3 mice. (B) Representative flow cytometric plots (left panel) and the percentage (right panel) of transfused CytoRed-positive Cd47−/− RBCs among total splenic RBCs at 2 hours after injection of the labeled cells in WT or XCR1DTRvenus recipient mice that had been treated with DTx 1 day previously. Data in the right panel are means ± SEM for 3 recipient mice. (C) Clearance of transfused Cd47−/− RBCs from peripheral blood of WT or XCR1DTRvenus recipient mice injected with the labeled cells at 1 day after DTx treatment. Data are means ± SEM for 3 recipient mice. (D) Representative flow cytometric plots for cDC subsets (left panel) and the absolute number of these cells (right panel) in the spleen of Sirpaf/f or SirpaΔDC mice. Data in the right panel are means ± SEM for 3 mice. (E) Representative flow cytometric plots (left panel) and the percentage (right panel) of transfused CFSE-positive Cd47−/− RBCs among total splenic RBCs at 2 hours after injection of the labeled cells in Sirpaf/f or SirpaΔDC recipient mice. Data in the right panel are means ± SEM for 3 recipient mice. (F) Clearance of transfused Cd47−/− RBCs from peripheral blood of Sirpaf/f or SirpaΔDC mice. Data are means ± SEM for 3 recipient mice. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001 by Student t test (A-B, D-E) or by 2-way repeated-measures ANOVA with Tukey’s multiple-comparison test (C,F). FSC-H, forward scatter height; NS, not significant.
Importance of SIRPα+ cDC2s for the splenic retention and rapid elimination of Cd47−/− RBCs in WT recipient mice. (A) Representative flow cytometric plots for cDC subsets (left panel) and the absolute number of these cells (right panel) in the spleen of WT or XCR1DTRvenus mice at 1 day after DTx treatment. Data in the right panel are means ± SEM for 3 mice. (B) Representative flow cytometric plots (left panel) and the percentage (right panel) of transfused CytoRed-positive Cd47−/− RBCs among total splenic RBCs at 2 hours after injection of the labeled cells in WT or XCR1DTRvenus recipient mice that had been treated with DTx 1 day previously. Data in the right panel are means ± SEM for 3 recipient mice. (C) Clearance of transfused Cd47−/− RBCs from peripheral blood of WT or XCR1DTRvenus recipient mice injected with the labeled cells at 1 day after DTx treatment. Data are means ± SEM for 3 recipient mice. (D) Representative flow cytometric plots for cDC subsets (left panel) and the absolute number of these cells (right panel) in the spleen of Sirpaf/f or SirpaΔDC mice. Data in the right panel are means ± SEM for 3 mice. (E) Representative flow cytometric plots (left panel) and the percentage (right panel) of transfused CFSE-positive Cd47−/− RBCs among total splenic RBCs at 2 hours after injection of the labeled cells in Sirpaf/f or SirpaΔDC recipient mice. Data in the right panel are means ± SEM for 3 recipient mice. (F) Clearance of transfused Cd47−/− RBCs from peripheral blood of Sirpaf/f or SirpaΔDC mice. Data are means ± SEM for 3 recipient mice. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001 by Student t test (A-B, D-E) or by 2-way repeated-measures ANOVA with Tukey’s multiple-comparison test (C,F). FSC-H, forward scatter height; NS, not significant.
As found previously, the number of cDC2s, but not that of cDC1s, is markedly reduced in the spleen of DC-specific SIRPα-deficient (SirpaΔDC) mice (Figure 5D).6,7 The splenic retention and rapid clearance of transfused Cd47−/− RBCs were significantly attenuated in SirpaΔDC mice compared with Sirpaf/f mice (Figure 5E-F). These results thus suggested that SIRPα-expressing CD4+ cDC2s, but not cDC1s, are important for the splenic retention and rapid elimination of Cd47−/− RBCs transfused into WT recipient mice.
Last, the status of other macrophages such as marginal zone macrophages (SIGNR1+ macrophages) and marginal metallophilic macrophages (CD169+ macrophages) in the spleen of CL-treated mice, DTx-treated CD11ciDTRΔ mice, and SirpaΔDC mice was evaluated. It was found that both single and double injections of CLs decreased the staining of SIGNR1+ cells and CD169+ cells in the spleen of mice (supplemental Figure 8), whereas the staining of SIGNR1+ cells and that of CD169+ cells in the spleen of DTx-treated CD11ciDTRΔ mice and SirpaΔDC mice was normal (supplemental Figure 9). Taken together with the results in this study, marginal zone macrophages and marginal metallophilic macrophages unlikely promote the splenic retention and clearance of Cd47−/− RBCs transfused into WT recipient mice.
Discussion
Conventional DCs play a major role in regulation of peripheral T-cell survival and homeostasis. Indeed, as antigen-presenting cells, cDCs promote the proliferation and differentiation of peripheral T cells. They also influence the survival of T cells in lymphoid organs through regulation of stromal cells, such as fibroblastic reticular cells.10,17 By contrast, it has remained unclear whether cDCs also contribute to control of the survival and homeostasis of circulating mature RBCs.
In this study, it is revealed that cDCs, in addition to RPMs, play an important role in the rapid elimination of transfused Cd47−/− RBCs from the bloodstream of WT recipient mice. The transfer of Cd47−/− RBCs was found to result in activation of both CD8+ cDC1s and CD4+ cDC2s in the spleen of WT recipients. The results of this study are consistent with previous observations that the activation of cDCs by transferred Cd47−/− RBCs results in stimulation of the adaptive immune system.8,9 However, a role for cDCs and their activation in the rapid elimination of transfused Cd47−/− RBCs from WT recipients has not previously been demonstrated. Phagocytosis by RPMs has been found to play a central role in the process by which Cd47−/− RBCs are eliminated from the body of WT mice.4,14 Indeed, Cd47−/− RBCs transfused into WT recipients were here confirmed to be rapidly incorporated into RPMs. In addition, the extent of phagocytosis of Cd47−/− RBCs by WT RPMs was much greater than that apparent for WT RBCs in vitro (supplemental Figure 10), consistent with previous findings.4 However, the depletion of RPMs alone by CLs only partly attenuated the rapid elimination of transfused Cd47−/− RBCs from the peripheral blood of WT recipient mice. It has been also demonstrated that the absence of RPMs by the knockout of transcription factor Spi-C did not affect the initial clearance of transfused Cd47−/− RBCs.18 These findings suggest that although RPMs play a pivotal role in the clearance of transferred Cd47−/− RBCs, other remaining cells may also remove these RBCs on behalf of RPMs. According to the results of experiments using DTx-treated CD11ciDTRΔ mice, cDCs mediate the rapid elimination of transfused Cd47−/− RBCs. Moreover, SIRPα-expressing CD4+ cDC2s, rather than CD8+ cDC1s, were found to play a pivotal role in this process.
The potential mechanism by which activated cDCs promote the rapid elimination of transfused Cd47−/− RBCs from the bloodstream of WT mice was also evaluated. It is confirmed that a small proportion of splenic cDCs incorporated transfused Cd47−/− RBC, consistent with previous findings.8 In addition, the transfusion of Cd47−/− RBCs into WT recipients resulted in the marked retention of the transfused cells in RP before their phagocytosis by RPMs. Moreover, depletion of cDCs, in particular, SIRPα-expressing CD4+ cDC2s, but not CD8+ cDC1s, prevented such retention of transfused Cd47−/− RBCs in the spleen of recipient mice. This retention of transfused Cd47−/− RBCs likely contributes to the rapid removal of these cells from the bloodstream to the spleen. In addition, the retention of RBCs in RP may facilitate effective phagocytosis of these cells by RPMs. The cDC-mediated retention of transfused Cd47−/− RBCs is therefore likely important for the rapid clearance of the transfused cells.
The expression of CD47 on RBCs is known to promote the survival of these cells.3,4 Previous studies have revealed that the expression of CD47 is reduced on aged RBCs.19,20 However, CD47 on aged RBCs has been found to increase an ability to bind to TSP-1 (Burger et al).21 Although CD47 is generally known to function as a “don’t eat me” signal, the CD47-TSP-1 interaction has been found to function as an “eat me” signal to induce phagocytosis of aged RBCs by RPMs.21 Taken together, these previous reports suggest that the decreased CD47 expression (a “don’t eat me” signal) and the increased CD47-TSP-1 interaction (an “eat me” signal) contribute to efficient phagocytosis of aged RBCs by RPMs. In addition, given the results of this study, the decreased CD47 expression on aged RBCs likely promotes the cDC-dependent retention of these cells. It is, thus, hypothesized that aged RBCs are retained and efficiently phagocytosed in the spleen by reducing CD47 expression.
Decreased CD47 expression on RBCs may promote the splenic retention and elimination of these RBCs not only in mice but also in humans. For example, the expression of CD47 is known to decrease in stored human RBCs,22 and some of stored RBCs are removed from the circulation within a few hours after the transfusion to humans.23 These removed RBCs from the circulation may be trapped in the spleen of humans. In addition, anti-CD47 antibodies that block the CD47-SIRPα interaction lead to reduce the number of RBCs (anemia) in a subset of patients.24,25 Given the results of this study, the inhibition of CD47-SIRPα interaction between RBCs and cDCs may induce the retention and clearance of RBCs in the spleen of human.
The precise mechanism by which the activation of cDC2s promotes the retention of transfused Cd47−/− RBCs in the spleen remains unknown. cDC2s are thought to be localized at the marginal zone and bridging channel of the spleen, the region near where the open-ended terminal arteries connect to RP from the white pulp.26,Cd47−/− RBCs, but not WT RBCs, therefore likely promote the activation of cDC2s through direct interaction, in turn resulting in modification of the RBCs and their retention in RP. Indeed, retention of RBCs in the spleen is thought to be attributable to a loss of cell deformability in anemic diseases, such as hereditary spherocytosis or malaria,27,28 both of which are characterized by marked congestion of RBCs in the spleen. Such abnormal or senescent RBCs with low deformability may be trapped in the splenic code as they are unable to pass through the endothelial slits of the venous sinuses, resulting in their phagocytosis by RPMs.28,29 However, any apparent morphological changes of Cd47−/− RBCs after their transfusion into WT mice were not detected.
In summary, in this study, a previously unrecognized role for cDCs in control of the survival and homeostasis of circulating mature RBCs was found. Further investigation will be necessary to clarify the molecular mechanism by which Cd47−/− RBCs activate cDCs of WT recipient mice, including how such activation promotes the splenic retention of Cd47−/− RBCs.
Acknowledgments
The authors thank Y. Matozaki, Y. Takase, and H. Okamura for technical assistance; K. Takeda from Osaka University for providing CD11c-iDTR mice; and A. Miyawaki (RIKEN) for giving permission for the use of mice carrying the Venus gene.
This work was supported by a Grant-in-Aid for Challenging Exploratory Research (22K19458 [T.M.]), Grants-in-Aid for Scientific Research (A) (21H04807 and 24H00617 [T.M.]), a Grant-in-Aid for Scientific Research (C) (24K10093 [T.T.]), and a Grant-in-Aid for Young Scientists (22K15395 [T.T.]) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by a Project for Promotion of Cancer Research and Therapeutic Evolution grant (22ama221304h0001 [T.M., T. Kotani, Y.S., and Y.M.]) from the Japan Agency for Medical Research and Development; by Moonshot R&D (JPMJMS2024 [R.N.]) from the Japan Science and Technology Agency; and by donation from T. Yamao (to Kobe University).
Authorship
Contribution: T.T., T. Kotani, and T.M. designed the research and wrote the paper; T.T. and H.E. performed the experiments; T.T. and T. Kotani prepared the figures; T.T., T. Kotani, H.E., Y.S., S.K., T.A., T.U., S.M., E.N., Y.M., T. Kaisho, and R.N. analyzed the data; and Y.S., S.K., H.E., T.A., Y.M., E.N., T. Kaisho, and R.N. reviewed the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Takashi Matozaki died on 12 May 2025.
Correspondence: Takenori Kotani, Division of Molecular and Cellular Signaling, Department of Biochemistry and Molecular Biology, Kobe University Graduate School of Medicine, 7-5-1 Kusunoki-cho, Chuo-ku, Kobe 650-0017, Japan; email: kotani@med.kobe-u.ac.jp.
References
Author notes
The authors agree to share original data and protocols available to other investigators without unreasonable restrictions. Original data and protocols are available on request from the corresponding author, Takenori Kotani (kotani@med.kobe-u.ac.jp).
The full-text version of this article contains a data supplement.

![Transfusion of Cd47−/− RBCs into WT mice induces cDC activation in parallel with incorporation of the transferred cells into RPMs and their clearance from the bloodstream. (A) Clearance of WT or Cd47−/− RBCs from peripheral blood of WT recipient mice. Data are means ± standard error of the mean (SEM) for 3 recipient mice. (B) Representative flow cytometric plots (left panel) and the percentage of CFSE-positive RPMs among total RPMs (right panel) for WT recipient mice at 2 hours after IV injection of CFSE-labeled WT or Cd47−/− RBCs. Data for mice that were not injected with RBCs are also revealed (RBC iv[−]). Data in the right panel are means ± SEM for 3 recipient mice. (C-E) Representative flow cytometric analysis of CD86 (C), CCR7 (D), and MHCII (E) expression (left panels) and quantitative data for MFI (right panels) for cDC subsets of WT recipient mice at 2 hours after IV injection of CFSE-labeled WT or Cd47−/− RBCs. Data for mice that were not injected with RBCs are also revealed (RBC iv[−]). Data in the right panels are means ± SEM for 3 recipient mice. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 by 2-way repeated-measures analysis of variance (ANOVA) with Tukey’s multiple-comparison test (A) or by 1-way ANOVA with Tukey’s multiple-comparison test (B-E). MFI, mean fluorescence intensity.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodrci/1/3/10.1016_j.brci.2025.100027/1/m_brci_rci-2025-000115-gr1.jpeg?Expires=1765290255&Signature=rcYu71ZWe2zTBVxqezfXu4qhQ1J~HAC0D1rU7Xal~O8I-nRKbWp-esHKZkVrmJjS0Jq8WXVCDfNpsjgILGfRZNKliyEx9wKeqG7hTK625aOffGltSOlmWJRacDclRErujDLzbXtkazH~GQqsO6uQjf4ria9y0XAg4ceT4bxIqpMcYfLNhkKqQghT53IIBTBuVCJEJSdoWUdwMMDj5PHHtsWPAIP8N6jxBO3eX6Dnni3dzK6ymsEUxZqImiYlgYIdCe8K6kA9DINaxo2UjO1gbTR4frP4UkP649N~xNd65ODWQVQtuhMKWKyrqD1vD4UAuS6B3e1gA2JLz3o1yxLmYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Two injections of CLs abolish the rapid elimination of transfused Cd47−/− RBCs from WT recipient mice. (A) Representative flow cytometric plots of RPMs (upper left panel) and cDCs (lower left panel) and the percentage of RPMs among total splenic cells (upper right panel) and that of cDCs among total splenic cells (lower right panel) on day 7 after treatment of mice with 50 μL of CLs (CL ×1) or PBS liposomes (PBS ×1) on day 0. Data in the right panels are means ± SEM for 4 mice. (B) Representative flow cytometric plots of RPMs (upper left panel) and cDCs (lower left panel) and the percentage of RPMs among total splenic cells (upper right panel) and that of cDCs among total splenic cells (lower right panel) on day 2 after treatment of mice with 100 μL of CLs (CL ×2) or PBS liposomes (PBS ×2) on day 0 and day 1. Data in the right panels are means ± SEM for 3 mice. (C) Clearance of transfused Cd47−/− RBCs from peripheral blood of WT recipient mice treated with CLs (CL ×1) or PBS liposomes (PBS ×1) as in panel A. Data for WT recipient mice injected with WT RBCs but not treated with liposomes (WT RBCs [no treatment]) are also revealed. The data in the lower panel are a more detailed representation of those in the upper panel. All data are means ± SEM for 3 recipient mice. (D) Clearance of transfused Cd47−/− RBCs from the peripheral blood of WT recipient mice treated with CLs (CL ×2) or PBS liposomes (PBS ×2) as in panel B. Data for WT recipient mice injected with WT RBCs but not treated with liposomes (WT RBCs [no treatment]) are also revealed. The data in the lower panel are a more detailed representation of those in the upper panel. All data are means ± SEM for 3 recipient mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 by Student t test (A-B) or by 2-way repeated-measures ANOVA with Tukey’s multiple-comparison test (C-D). NS, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodrci/1/3/10.1016_j.brci.2025.100027/1/m_brci_rci-2025-000115-gr2.jpeg?Expires=1765290255&Signature=VZzy4A3-TE~H7~qXkx8wA~~NrHtyTyDc9xLu~zVKKMTKjvg3PuVpCxuOoGC8Hsivne0iyAOpbbEU~r4gbh1PgOQMpCjKbMkNZSWhjiHn47VQgEMo90pyiKsyYotTl8JEFG1QZyj4IqGbS4lbUHjalfs-63AuWvOgEZXlq7qpibuuLjUCQmCI6221O74xj0BM5~WD7Wmk4RzGrAsE5SP88t0LGwTvyPdEndvhBOFdW8sp108-rQ2nR3aFf8F92IXqbxZ6k~P0ZY5wctSNkP0CaTloJSgy~jkynT-glPWrl6RuqilSxHWTTda~8qH5Kvqm889T7r8TwFOVrvVkS6tWzg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)