TO THE EDITOR:
Sickle cell disease (SCD) is characterized by recurrent sickling and hemolysis of red blood cells, resulting in vaso-occlusive episodes (VOEs).1 When severe, patients with VOE may require hospitalization for treatment of severe pain and management of complications such as acute chest syndrome (ACS), stroke, and venous thromboembolism.1-9 Although inflammatory markers, such as C-reactive protein, and hemolysis indices, such as lactate dehydrogenase, are routinely used as nonspecific biomarkers to predict the development of VOEs, clinicians lack systems to reliably quantify disease trajectory and outcomes in the inpatient setting for prognostication.10
Prior studies have shown that absolute eosinophil count (AEC) is higher among patients with SCD than those without SCD.2,11,12 Moreover, eosinophils isolated from the peripheral blood of patients with SCD produce greater quantities of reactive oxygen species and exhibit enhanced adhesive properties, higher levels of inflammatory chemokines, and increased spontaneous migration relative to eosinophils from controls.2,11,13-15 Upon disease-modifying treatment with hydroxyurea (HU), patients with SCD were found to have reduced eosinophil degranulation and reduced frequency of VOE compared to those not treated with HU.2,11
Because eosinophil activation appears to reflect disease severity at the molecular level, we sought to evaluate whether AEC could reliably predict outcomes among patients with SCD hospitalized with VOEs.
This multicenter retrospective cohort study was performed using the Premier Healthcare Database (1 October 2022 and 30 September 2024), an enhanced administrative database that includes demographics, claims codes, and billing data from ∼25% of US hospitalizations. A subset of hospitals in Premier Healthcare Database also contribute laboratory data to the database and were used for this study. Included patients were aged 10 to 70 years, had an SCD VOE International Classification of Diseases, 10th Revision diagnosis code (D57.00, D57.02, D57.04, and D57.09) present at the time of admission, and had at least 1 blood AEC between 0 and 5000 cells per μL recorded on the first day of admission. For patients with multiple AECs measured on the day of admission, the first was used in analyses. We excluded patients with ACS, venous thromboembolism, or ischemic stroke at the time of admission because we were interested in the development of these diseases as outcomes during hospitalization.
The exposure of interest was AEC on the day of admission. The primary outcome was hospital length of stay (LOS); secondary outcomes were the composite of ACS, venous thromboembolism, or ischemic stroke (ie, severe complications from SCD vaso-occlusive episodes) and hospital mortality. We chose hospital LOS as the primary outcome, because increased hospitalization duration is likely to be the end result of uncontrolled VOE-associated pain or of complications from VOE. From each patient, we also extracted covariables including demographics (age and sex), hemoglobin concentration on the day of admission, HU use on the day of admission, organ dysfunction, and comorbidities present on admission.16,17 Organ dysfunction and comorbidity severity were defined using Angus algorithms and Gagne combined comorbidity scores, respectively.
We first used Spearman correlation to assess correlation between AEC and hospital LOS. We then used median regression to quantify associations (bootstrapped 95% confidence intervals [CIs]) between AEC and hospital LOS. We used Akaike Information Criterion (AIC) to assess changes in model goodness of fit from a baseline median regression model including just covariables to a model with covariables and AEC. Thus, a decrease in AIC when adding AEC to the model suggested that model fit improved with the addition of AEC. Logistic regression was used to quantify the association between AEC and secondary outcomes. Complete case analysis was used during modeling. Statistical analyses were performed in R (version 4.0.5). Alpha was set at .05 for all comparisons. This study was designated not human subjects research by the Institutional Review Board at Boston University (H-41795) and followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.18
Among 4690 patients with SCD VOE and no exclusions, 3078 had at least 1 AEC on the first day of admission. The median age was 31 years (interquartile range [IQR], 24-38; Table 1). The median hemoglobin at admission was 8.3 g/dL (IQR, 7.3-9.5); 10 patients had missing hemoglobin concentrations. A total of 799 patients (26.0%) received HU on the day of hospital admission. In addition, <0.3% of the study sample were being treated with other disease-modifying drugs (ie, crizanlizumab and voxelotor) at the time of admission.
Characteristics of included patients
| Characteristic . | Patients with SCD VOE (N = 3078) . |
|---|---|
| Age, median (IQR)∗, y | 31 (24-38) |
| Sex, n (%) | |
| Male | 1379 (44.8) |
| Female | 1699 (55.2) |
| Organ dysfunctions present on admission, n (%) | |
| Cardiac | 36 (1.2) |
| Respiratory | 91 (3.0) |
| Neurologic | 22 (0.7) |
| Hematologic | 112 (3.6) |
| Hepatic | 3 (0.1) |
| Renal | 151 (4.9) |
| Comorbidity Severity Score, points (IQR) | 1 (0-2) |
| Hemoglobin concentration on the day of admission, median (IQR)∗,†, g/dL | 8.3 (7.3-9.6) |
| HU use on day of admission, n (%) | 799 (26.0) |
| Characteristic . | Patients with SCD VOE (N = 3078) . |
|---|---|
| Age, median (IQR)∗, y | 31 (24-38) |
| Sex, n (%) | |
| Male | 1379 (44.8) |
| Female | 1699 (55.2) |
| Organ dysfunctions present on admission, n (%) | |
| Cardiac | 36 (1.2) |
| Respiratory | 91 (3.0) |
| Neurologic | 22 (0.7) |
| Hematologic | 112 (3.6) |
| Hepatic | 3 (0.1) |
| Renal | 151 (4.9) |
| Comorbidity Severity Score, points (IQR) | 1 (0-2) |
| Hemoglobin concentration on the day of admission, median (IQR)∗,†, g/dL | 8.3 (7.3-9.6) |
| HU use on day of admission, n (%) | 799 (26.0) |
A total of 116 patients were 10 to 16 years old, and 651 patients were 40 to 70 years old.
Missing in 12 patients.
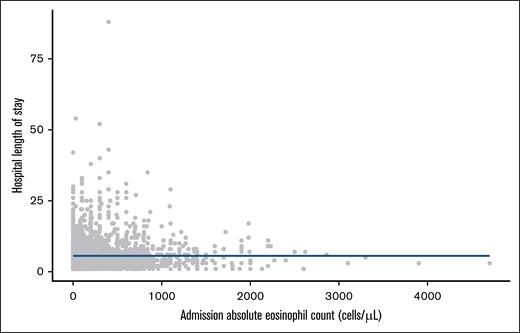
The overall median AEC was 200 cells per μL (IQR, 90-400), and the overall median hospital LOS was 4 days (IQR, 3-7). The relationship between AEC and hospital LOS is shown in Figure 1. There was no correlation between AEC and hospital LOS (Spearman ρ, –0.007; P = .70), and there was no association between AEC and hospital LOS in the unadjusted (β, 0.0 per 1000 cells per μL [95% CI, 0.0-0.0]; P = .65) or adjusted (β, –0.1 per 1000 cells per μL [–0.4 to 0.2]; P = .49) median regression models. Adding AEC to a model including just covariables increased the AIC from 17 265 to 17 271. There were no associations between AEC and hospital mortality (13 deaths; adjusted odds ratio, 1.00 per 1000 cells per μL [95% CI, 0.995-1.001]; P = .36) or between AEC and the composite of ACS, venous thromboembolism, or ischemic stroke (32 events; adjusted odds ratio, 1.03 [95% CI, 0.32-2.33]).
Association between AEC on admission and hospital LOS. Each dot represents a patient. The blue line corresponds to a fitted penalized natural spline from a generalized additive model for the associations between AEC and hospital LOS.
Association between AEC on admission and hospital LOS. Each dot represents a patient. The blue line corresponds to a fitted penalized natural spline from a generalized additive model for the associations between AEC and hospital LOS.
In a multicenter retrospective study of patients hospitalized with SCD VOE, admission AEC was not associated with hospital LOS, hospital mortality, or complications of severe VOE. These results, in contrast to preclinical data, do not support AEC as an indicator of disease severity among hospitalized patients with SCD VOE.
This study has limitations. We did not have access to prehospitalization AECs to compare with AECs obtained during admission. Moreover, our International Classification of Diseases coding system may be unable to appropriately qualify those with severe disease, because the distinction between a complicated VOE and an uncomplicated VOE may require expert clinician opinion beyond that which can be captured in an administrative database. Finally, we included only data from hospitalized patients in this study and excluded those in the outpatient setting; outpatients could potentially exhibit eosinophilia before presenting with VOE.
Our study did not show a correlation between AEC and acuity of SCD VOE among hospitalized patients. The discrepancy between preclinical data and our findings may be a reflection of setting, because our data were obtained exclusively from hospitalized patients. In light of these findings, we posit that the AEC may be a reflection of SCD disease control and unreliable in the inpatient setting. Future studies on AEC in the outpatient setting as a predictor of hospitalization, VOE, ACS, or thromboembolic events may prove fruitful. Furthermore, the quantitative AEC may not directly correspond to eosinophil activity. As a result, we advocate for further translational research into SCD prognostic tools and outcomes.
Acknowledgments: This work was supported by the American Heart Association Grant 24CDA1267699. D.N.D. is supported by the National Institutes of Health (grant 19 R38HL143584 PRIMER [Promoting Research in Medical Residency]).
Contribution: N.A.B. contributed to acquisition, analysis, or interpretation of data including statistical analyses; N.A.B. and K.L.M. supervised the study; and all authors contributed to study concept and design, and critical revision of the manuscript for important intellectual content, and had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Grace M. Ferri, Hematology/Oncology, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215; email: gferri@bidmc.harvard.edu.
References
Author notes
G.M.F. and R.E.P.A. are joint first authors.
N.A.B. and K.L.M. are joint senior authors.
All data used in this study are available from the corresponding author, Grace M. Ferri (gferri@bidmc.harvard.edu), on reasonable request.